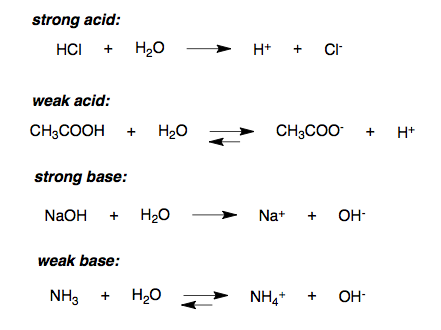
My mate and I are making a water base on realms. Any ideas for the roof? We've been stumped for a couple days now : r/Minecraft

Acids and Bases. Dissociation of Strong Bases Strong bases are metallic hydroxides Group I hydroxides (NaOH, KOH) are very soluble Group II hydroxides. - ppt download
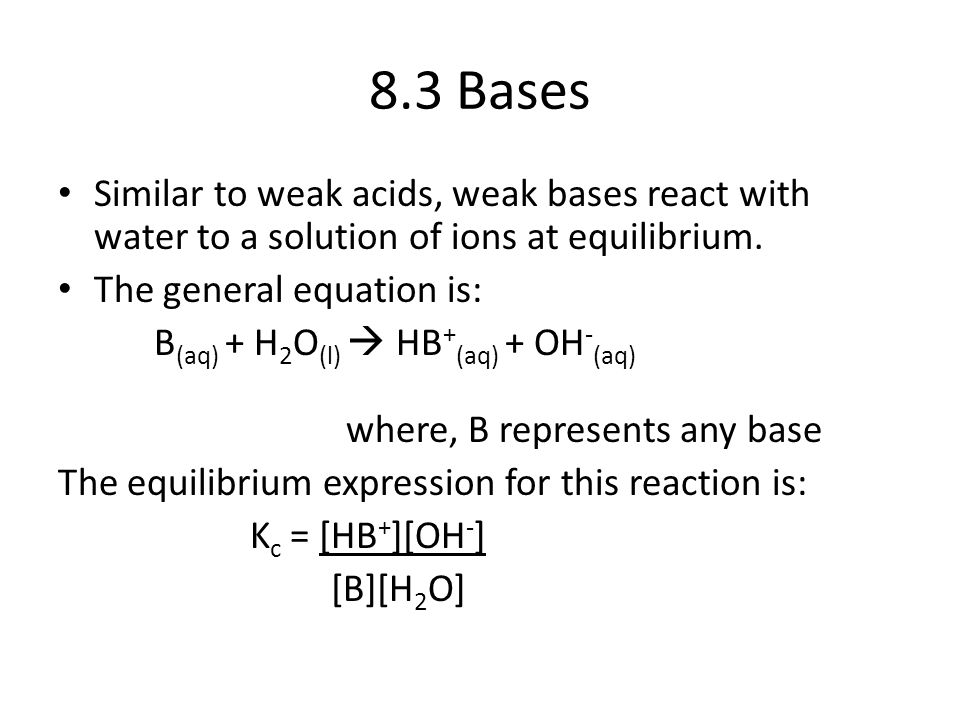
8.3 Bases Similar to weak acids, weak bases react with water to a solution of ions at equilibrium. The general equation is: B(aq) + H2O(l) HB+(aq) + - ppt video online download

Properties of Water Overview & Examples | Is Water an Acid or a Base? - Video & Lesson Transcript | Study.com


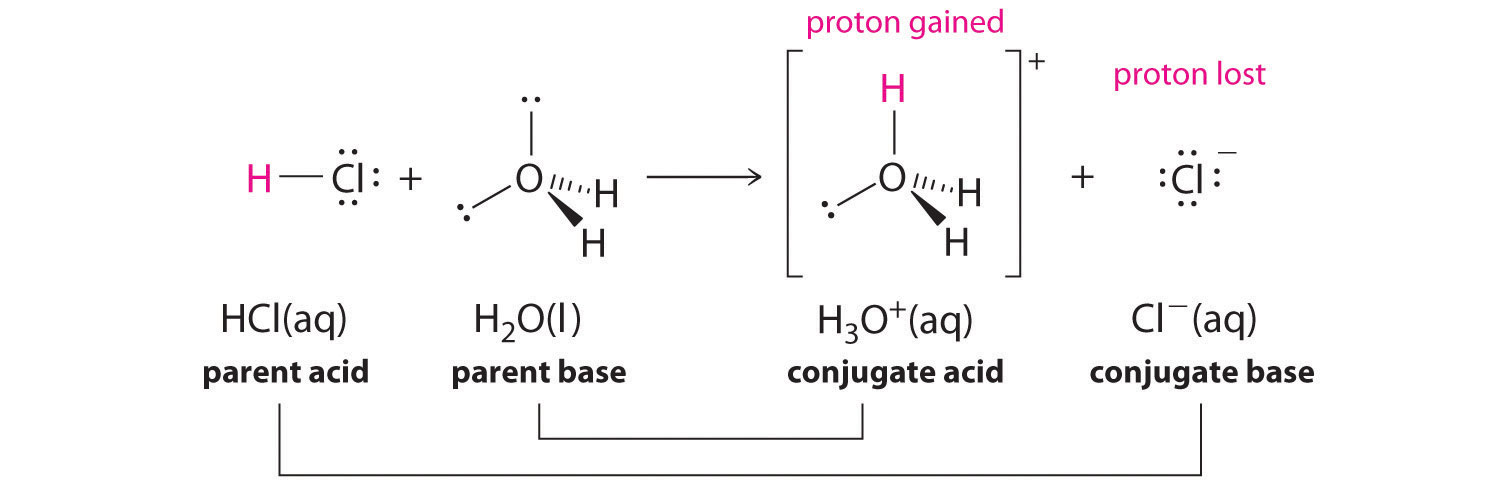




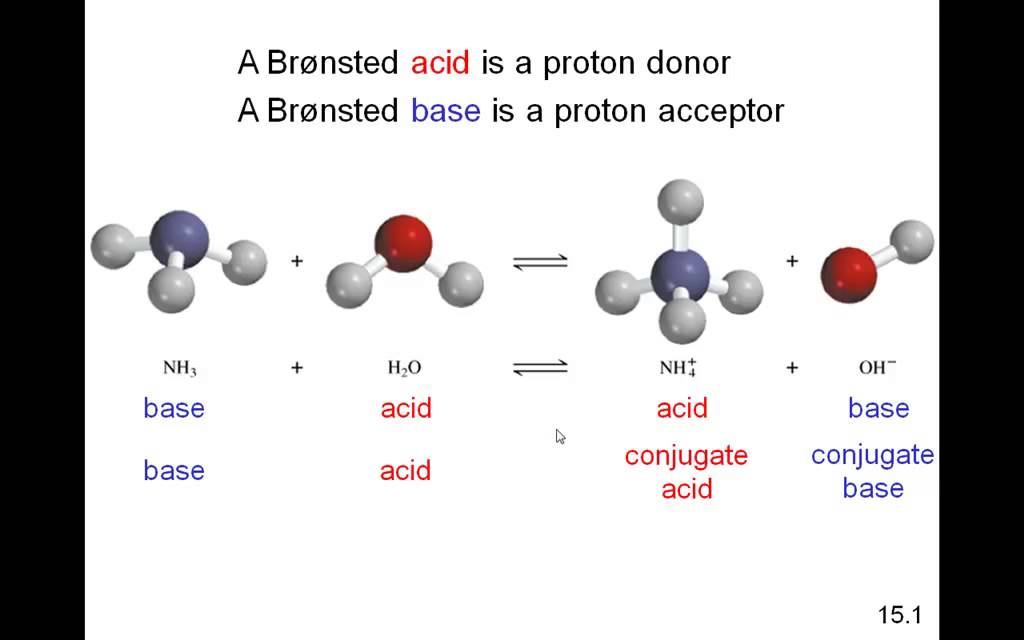




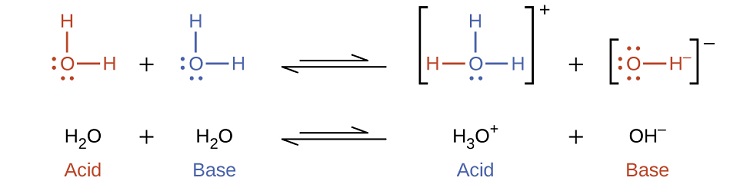
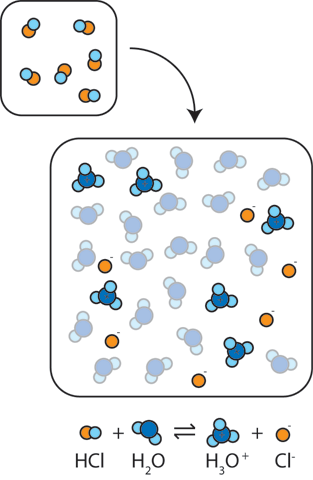
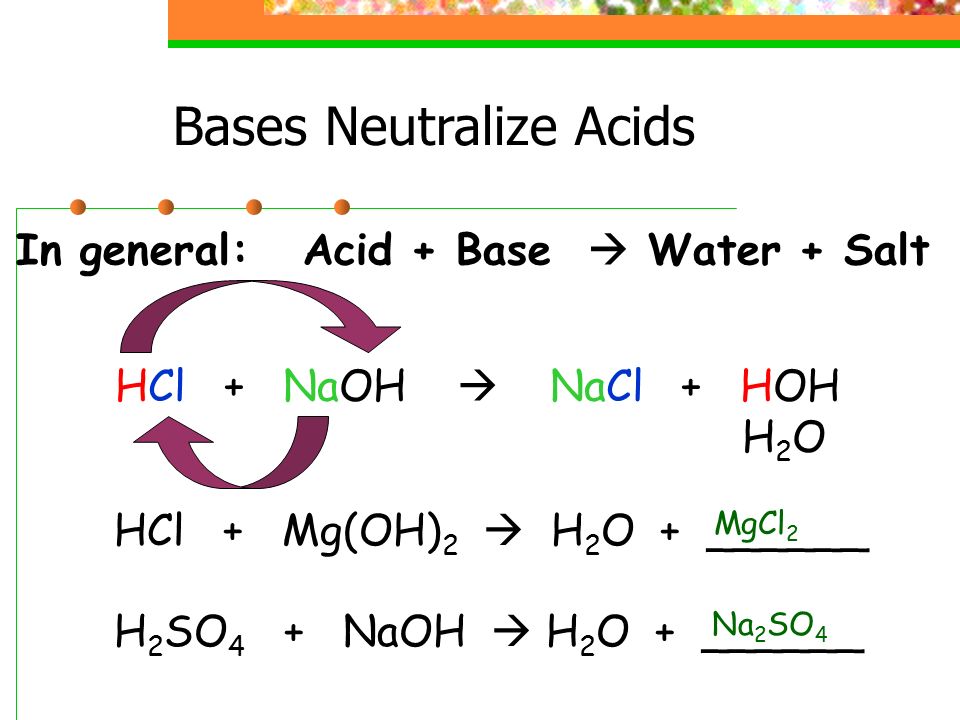


-in-water-01.jpg)