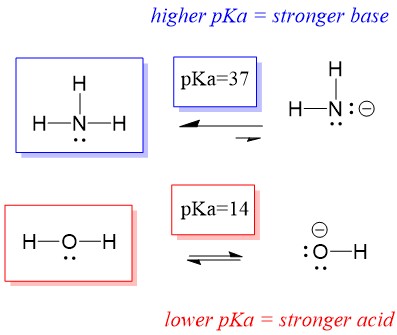
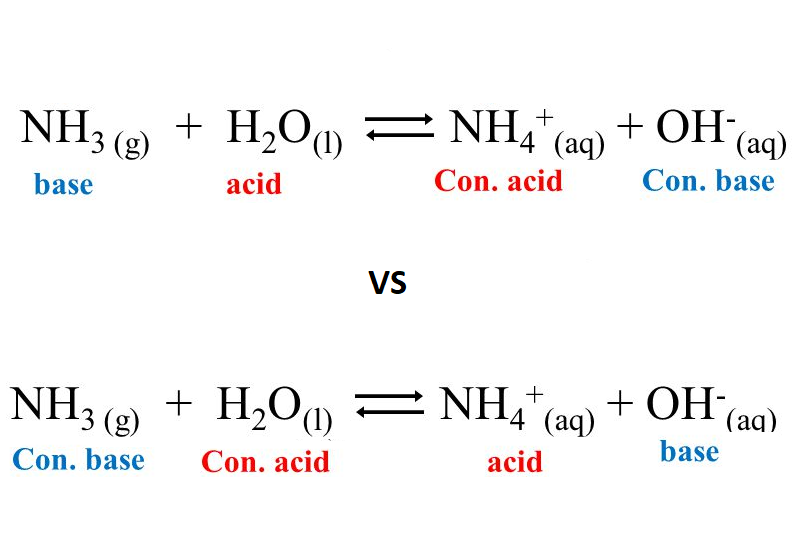
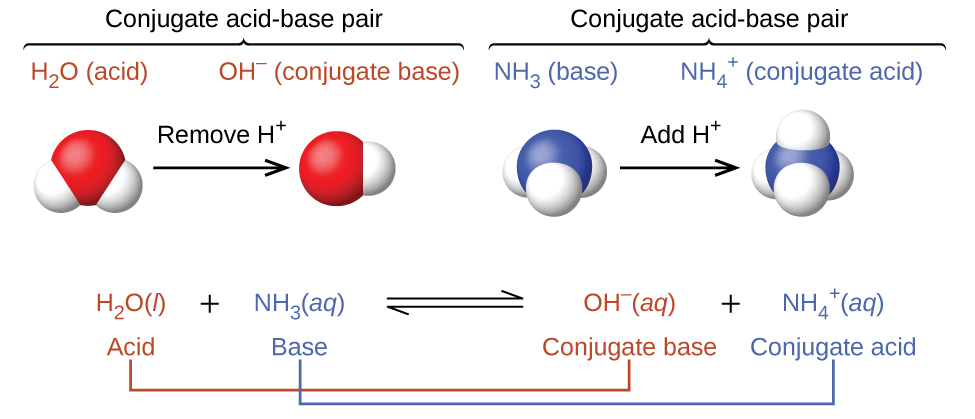
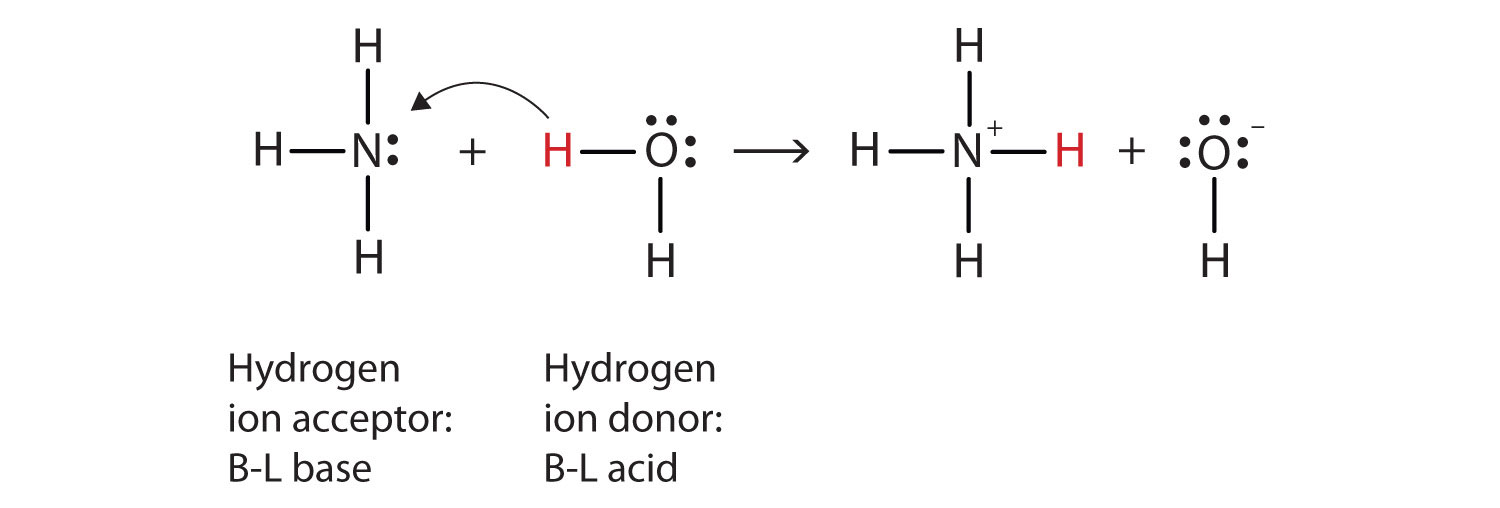
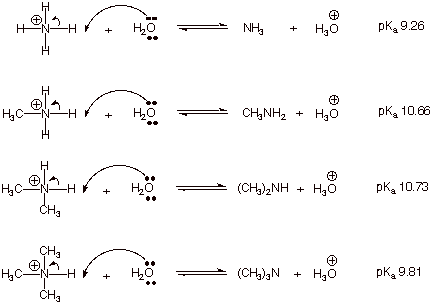Explain the differences between the Bronsted-Lowry and the Lewis acid-base theories, using the formation of the ammonium ion from ammonia and water to illustrate your points. | Homework.Study.com

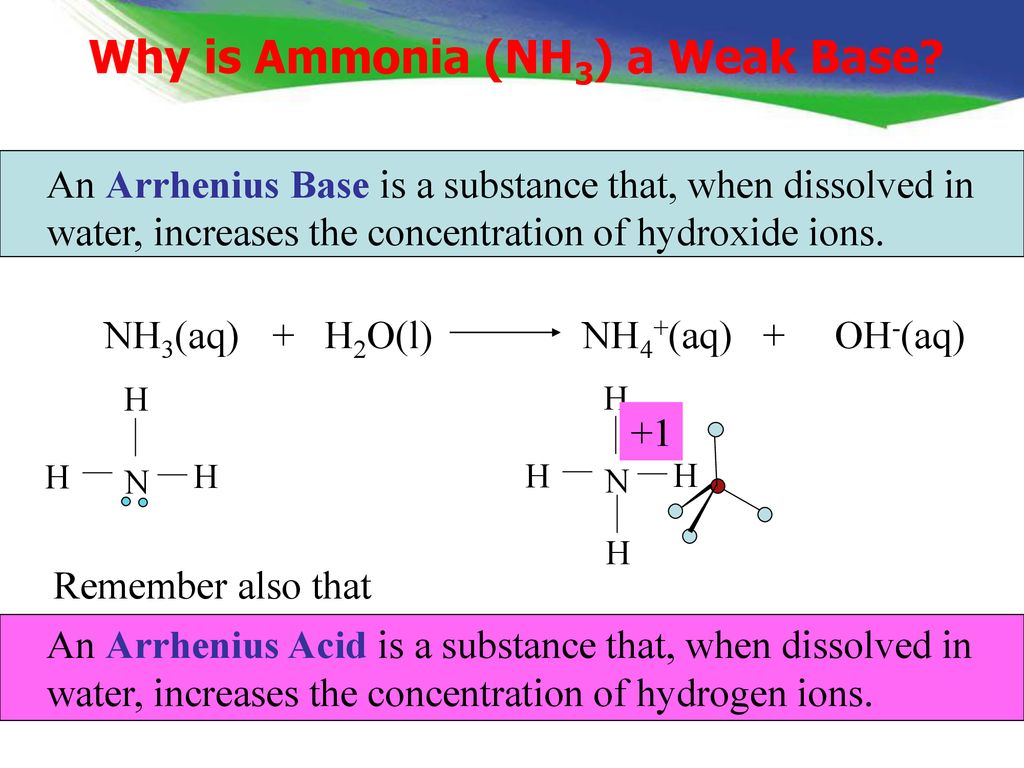
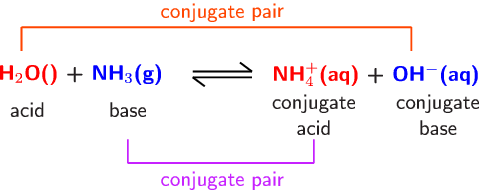
a) Explain why ammonia acts as a weak base in water. (b) Write a balanced chemical equation for the reaction between ammonia and water. | Homework.Study.com

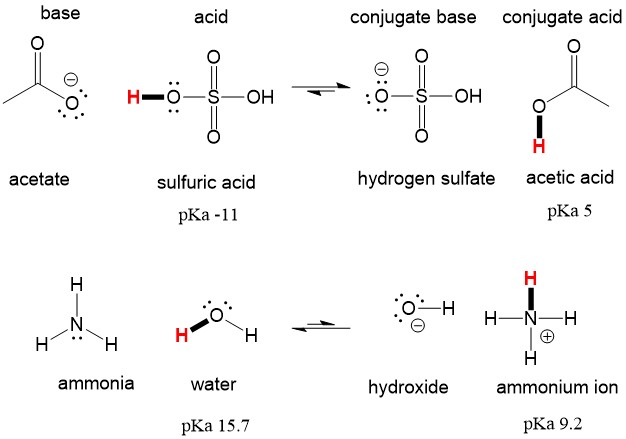
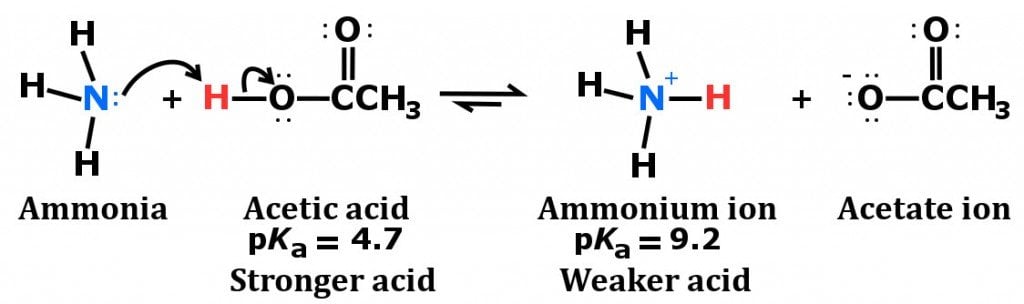
organic chemistry - Why In This Reaction Acetic Acid is strong acid and NH3 is strong base ?please explain in details and thanks for answer - Chemistry Stack Exchange